A directing group free Pd(ii)-catalysed desulfitative C6-arylation of 2-pyridone using an arylsulfonyl chloride†
Abstract
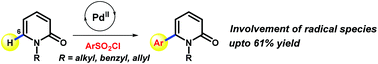
A Pd(II)-catalysed direct desulfitative arylation was realized at the C6-position of the 2-pyridone scaffold. Aryl sulfonyl chloride was used as an alternative arylating agent. The required site-selectivity occurred without the strategic installation of a heteroatom containing directing group. Preliminary mechanistic studies revealed that radical species were involved during this process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...