Rapid access to 3-indolyl-1-trifluoromethyl-isobenzofurans by hybrid use of Lewis/Brønsted acid catalysts†
Abstract
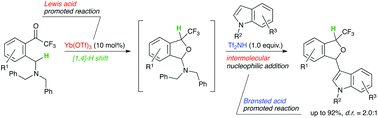
We report herein a rapid access to 3-indolyl-1-trifluoromethyl-isobenzofurans via a [1,4]-hydride shift/cyclizatin/intermolecular nucleophilic addition reaction sequence. In this process, a Lewis acid promoted internal redox reaction ([1,4]-hydride shift/cyclization) followed by a Brønsted acid promoted intermolecular reaction (generation of cyclic oxonium cation/intermolecular Friedel–Crafts reaction) occurred to give various 3-indolyl-1-trifluoromethyl-isobenzofurans in good chemical yields.
- This article is part of the themed collections: Hybrid Catalysis and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...