Solid-phase synthesis and evaluation of tumour-targeting phenylboronate-based MRI contrast agents†
Abstract
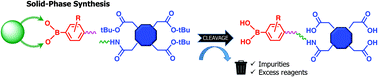
Paramagnetic macrocycles functionalized with phenylboronic moieties have proven to be interesting for MRI applications based on their ability to recognize cancer cells and generate local contrast. However, full use of the potential of this class of compounds is hampered by laborious and inefficient synthetic and, especially, purification procedures. The amphiphilic character of water-soluble phenylboronates renders them difficult compounds to be prepared through conventional solution synthesis due to the tendency to aggregate and form adducts with other nucleophiles. The new strategy described herein exploits the advantage of solid-phase synthesis with the application of DEAM-PS resin for anchorage and the subsequent simplified derivatization of boronates. GdDOTA-EN-PBA and its fluorinated analogue GdDOTA-EN-F2PBA were synthesized in a much easier, faster and economically convenient way to achieve good yields and purity. Furthermore, the effect of electron-withdrawing fluorine atoms on the aromatic ring of the latter compound was investigated by comparing the physico-chemical properties of both compounds as well as their binding affinity towards melanoma cancer cells.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...