Synthesis of organoselenyl isoquinolinium imides via iron(iii) chloride-mediated tandem cyclization/selenation of N′-(2-alkynylbenzylidene)hydrazides and diselenides†
Abstract
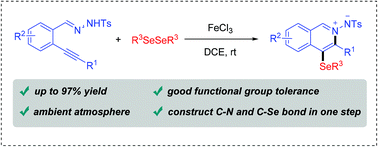
This report describes the synthesis of organoselenyl isoquinolinium imides through a tandem cyclization between N′-(2-alkynylbenzylidene)hydrazides and diselenides. The reaction was carried out at room temperature under an ambient atmosphere using cheap iron(III) chloride as the metallic source. The strategy shows good tolerance to a broad range of N′-(2-alkynylbenzylidene)hydrazides and diselenides, and forms C–N and C–Se bonds in one step. The obtained product is further transformed into a bioactive H-pyrazolo[5,1-a]isoquinoline skeleton easily via a silver catalyzed [3 + 2] cycloaddition.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...