A mechanistic study on Cu(i) catalyzed carboxylation of the C–F bond with CO2: a DFT study†
Abstract
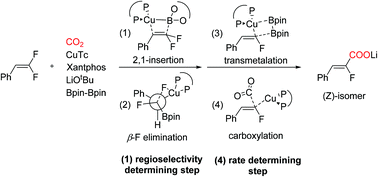
The Cu(I) catalyzed carboxylation of the C–F bond recently reported by Yu and co-workers is an excellent method for the construction of complex fluoroacrylate compounds with high regioselectivity. In the present study, theoretical calculations were carried out to investigate the detailed mechanism of the catalytic cycle and the origin of regioselectivity. The calculation results reveal that the overall catalytic cycle proceeds via the migratory insertion of difluoroalkene on the boryl–Cu(I) species, syn β-F elimination, transmetalation, and carboxylation steps. The rate determining step is the carboxylation step, and the migration insertion is the regioselectivity determining step. The regioselectivity for 2,1-insertion is consistent with Yu's experiment, and is determined by both steric and electronic effects.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...