Modular quadruplex–duplex hybrids as biomolecular scaffolds for asymmetric Michael addition reactions†
Abstract
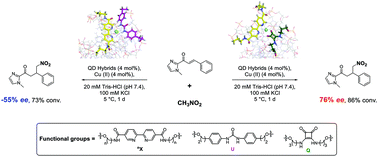
Asymmetric synthesis based on DNA scaffolds has been actively exploited because of the advantages of DNA such as diverse tertiary structures, chemical stability, and easy handling. Since duplex DNA-based hybrid catalysts have demonstrated this remarkable capability, efforts have been made to investigate new biomolecular scaffolds. Herein, we report modular quadruplex–duplex (QD) hybrid DNA catalysts containing bipyridine ligands and hydrogen donor moieties. The conformation, thermal stability, and metal-binding ability of modified QD hybrid DNA were characterized using spectroscopy. The QD hybrid-based DNA catalysts were successfully applied to asymmetric Michael addition reactions (86% conversion and 76% ee). This study describes a new type of DNA hybrid catalyst produced by the construction of a cooperative active site with a Lewis acid and a H-bond donor.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...