MnO2 as a terminal oxidant in Wacker oxidation of homoallyl alcohols and terminal olefins†
Abstract
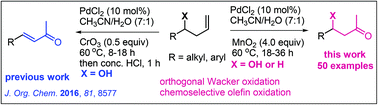
Efficient and mild reaction conditions for Wacker-type oxidation of terminal olefins of less explored homoallyl alcohols to β-hydroxy-methyl ketones have been developed by using a Pd(II) catalyst and MnO2 as a co-oxidant. The method involves mild reaction conditions and shows good functional group compatibility along with high regio- and chemoselectivity. While our earlier system of PdCl2/CrO3/HCl produced α,β-unsaturated ketones from homoallyl alcohols, the present method provided orthogonally the β-hydroxy-methyl ketones. No overoxidation or elimination of benzylic and/or β-hydroxy groups was observed. The method could be extended to the oxidation of simple terminal olefins as well, to methyl ketones, displaying its versatility. An application to the regioselective synthesis of gingerol is demonstrated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...