Planar chiral palladacycle precatalysts for asymmetric synthesis†
Abstract
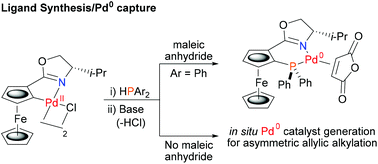
Chiral non-racemic palladacycles were employed as precatalysts for Pd(0) mediated asymmetric synthesis. Addition of HPAr2/base to a ferrocenyloxazoline planar chiral palladacycle resulted in ligand synthesis and palladium capture to give a bidentate Phosferrox/Pd(0) complex. A series of these complexes were generated in situ and applied successfully as catalysts for asymmetric allylic alkylation.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...