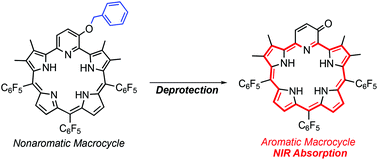
Deprotection of a benzyl unit induces a 22π aromatic macrocycle of 3-oxypyripentaphyrin(0.1.1.1.0) with strong NIR absorption†
Abstract
We report aromaticity switching from a 6π pyridine ring to a 22π macrocyclic ring of 3-oxypyripentaphyrin(0.1.1.1.0). This system has potential applications in photodynamic therapy owing to macrocyclic aromaticity being selectively induced by protecting group removal and strong absorption bands produced in the NIR region especially in methanol.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...