Route exploration and synthesis of the reported pyridone-based PDI inhibitor STK076545†
Abstract
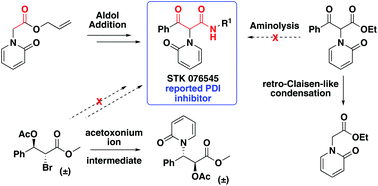
The enzyme protein disulfide isomerase (PDI) is essential for the correct folding of proteins and the activation of certain cell surface receptors, and is a promising target for the treatment of cancer and thrombotic conditions. A previous high-throughput screen identified the commercial compound STK076545 as a promising PDI inhibitor. To confirm its activity and support further biological studies, a resynthesis was pursued of the reported β-keto-amide with an N-alkylated pyridone at the α-position. Numerous conventional approaches were complicated by undesired fragmentations or rearrangements. However, a successful 5-step synthetic route was achieved using an aldol reaction with an α-pyridone allyl ester as a key step. An X-ray crystal structure of the final compound confirmed that the reported structure of STK076545 was achieved, however its lack of PDI activity and inconsistent spectral data suggest that the commercial structure was misassigned.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...