Synthesis of trifluoromethylthioesters from aldehydes via a visible light-promoted radical process†
Abstract
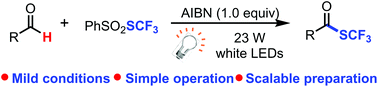
We report herein an efficient, economical, and scalable trifluoromethylthiolation of aldehydes to generate trifluoromethylthioesters via a visible light-promoted radical process. The transformation features cheap reagents, simple operation, a broad substrate scope, and especially no metal involved in the reaction. Trifluoromethylthiolations of several complex aldehyde-containing bioactive compounds have been realized; thus the approach has the potential to be an important tool for the late-stage functionalization of advanced synthetic intermediates and bioactive molecules, and should have many applications in medicinal chemistry.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...