Indirect synthetic route to α-l-fucosides via highly stereoselective construction of α-l-galactosides followed by C6-deoxygenation†
Abstract
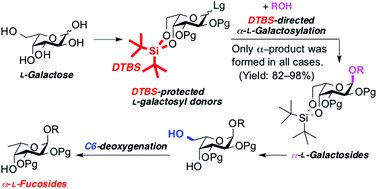
We developed an indirect synthetic method for α-L-fucosides. Based on the fact that L-fucose is 6-deoxy-L-galactose, our strategy consists of the stereoselective construction of α-L-galactoside and its conversion to α-L-fucoside via C6-deoxygenation. The formation of α-L-galactoside is strongly directed using 4,6-O-di-tert-butylsilylene(DTBS)-protected L-galactosyl donors. The DTBS-directed α-L-galactosylation showed broad substrate applicability along with excellent coupling yield and α-selectivity. In the C6-deoxygenation of α-L-galactosides, the Barton–McCombie reaction facilitated the conversion to L-fucosides with good yield. To demonstrate the applicability of our method, we synthesized naturally occurring α-L-fucosides.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...