Selective functionalization of 6-amino-6-methyl-1,4-perhydrodiazepine for the synthesis of a library of polydentate chelators†
Abstract
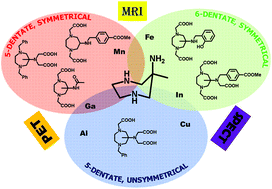
Polydentate chelators are an important part of an imaging probe, which consists of an agent that usually produces signals for imaging purposes connected to a targeting moiety. The goal of this study was to set up a generic protocol to prepare a library of polydentate ligands having a 6-amino-6-methyl-1,4-perhydrodiazepine (AMPED) core and able to chelate metal ions of interest for various diagnostic imaging techniques, including Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT). These ions, among which we can include Mn(II), Cu(II), Al(III) or Ga(III), require penta- or hexa-dentate chelators for this purpose, and the AMPED scaffold has considerable potential to support various pendant arms for coordination of such ions. AMPED already has three amino nitrogen donors; thus, only two or three additional arms should be introduced to obtain penta- or hexa-dentate systems. This condition implies that symmetrical or asymmetrical structures have to be developed, depending on the functionalization of cyclic and exocyclic amines. Starting from easily available materials, we have designed a convenient protocol for the preparation of multiple AMPED-based ligands endowed with different characteristics, several of which were synthesized as examples.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...