Recent advances in the oxime-participating synthesis of isoxazolines
Abstract
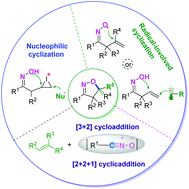
Isoxazoline compounds are used as important intermediates for the synthesis of organic molecules, which are widely used in the chemical and life science industries. Oxime-participating cyclization has emerged as an efficient strategy for the construction of isoxazolines. This review is devoted to highlighting the main achievements (since 2010) in the development of methodologies for the synthesis of isoxazolines. According to the reaction mechanism, the oxime-participating synthesis of isoxazolines can be mainly classified into four reaction types: iminoxyl radical-initiated intramolecular cyclization, intermolecular radical addition-initiated cyclization, intramolecular nucleophilic cyclization, and [3 + 2] cycloaddition. Meanwhile, miscellaneous examples are also illustrated, such as [2 + 2 + 1] cycloaddition. Representative reactions will be discussed for each of the highlighted synthetic strategies. In addition, the enantioselective synthesis of isoxazolines is also illustrated in this review.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...