Nitroepoxide ring opening with thionucleophiles in water: synthesis of α-xanthyl ketones, β-keto sulfones and β-keto sulfonic acids†
Abstract
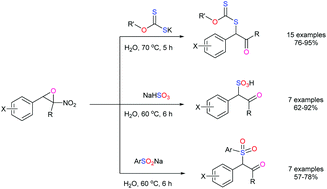
Nitroepoxide ring opening with thionucleophiles such as potassium xanthates, sodium aryl sulfinates and sodium bisulfite in water is investigated. It provides a direct and green route for the synthesis of α-xanthyl-α-aryl-2-propanones (P2P-xanthate derivatives), β-keto sulfones and β-keto sulfonic acids. High to excellent yields, performing the reaction in water under catalyst-free conditions, and easy work-up are the main advantages of this protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...