Palladium-catalyzed three-component cascade arylthiolation with aryldiazonium salts as S-arylation sources†
Abstract
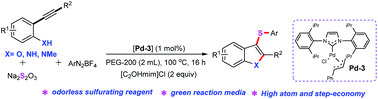
A novel and efficient palladium-catalyzed three-component cascade cyclization/arylthiolation has been developed for the assembly of diverse 3-sulfenylindole and 3-sulfenylbenzofuran derivatives from 2-alkynylamines and 2-alkynylphenols, aryldiazonium salts, and Na2S2O3 under aerobic conditions with PEG-200 as an environmentally benign medium. The current study features exceptional functional group tolerance without additional ligands, oxidants or silver salts, and eco-friendly mild reaction conditions. The ionic liquid [C2OHmim]Cl plays a crucial role in this protocol as an environmentally friendly additive. Notably, this procedure represents the first example of the use of aryldiazonium salts as direct S-arylation sources in this type of chemical transformation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...