Efficient copper(i)-catalyzed oxidative intermolecular 1,2-estersulfenylation of styrenes with peroxyesters and disulfides†
Abstract
A simple and practical method for the synthesis of thio-substituted esters through copper(I)-catalyzed intermolecular 1,2-estersulfenylation of styrenes with peroxyesters and disulfides was developed. In this transformation, two new C–S bond and C–O bond were constructed simultaneously under a copper catalyst system, and the transformation exhibits a broad substrate scope and good functional group compatibility. In addition, this method can also be applied to arylthiols. It should be noted that peroxyesters not only acted as nucleophilic reagents but also as oxidants.
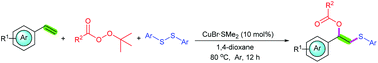
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...