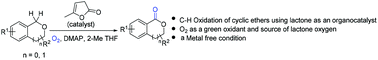
α-Angelica lactone catalyzed oxidation of benzylic sp3 C–H bonds of isochromans and phthalans†
Abstract
A metal-free organocatalytic system has been developed for highly efficient benzylic C–H oxygenations of cyclic ethers using oxygen as an oxidant. This oxidation reaction utilizes α-angelica lactone as a low cost/low molecular weight catalyst. The optimized reaction conditions allow the synthesis of valued isocoumarins and phthalides from readily available precursors in good yields. Mechanistic studies indicate that the reaction pathway likely involves a radical process via a peroxide intermediate.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...