Synthesis of protected α-amino acids via decarboxylation amination from malonate derivatives†
Abstract
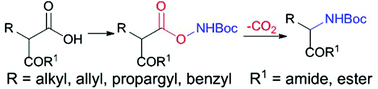
A general and efficient strategy for the synthesis of protected α-amino acids is reported. The method uses malonate derivatives as the starting materials and Cs2CO3 as a base at 60 degrees, giving α-amino acid derivatives in moderate yields by releasing CO2. This methodology shows broad substrate scope (primary and secondary acids), excellent functional group tolerance and high efficiency to give the desired products under mild reaction conditions. It also allows the construction of β and γ-amino acids and other unnatural products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...