Synthesis of glycosyl sulfoximines by a highly chemo- and stereoselective NH- and O-transfer to thioglycosides†
Abstract
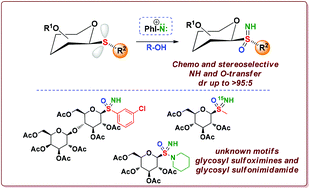
A synthesis of unprecedented and stable glycosyl sulfoximines is reported. The developed strategies represent the first example of highly stereoselective sulfoximine formation directly from thioglycosides. This synthetic protocol has been tested on several β-thioglycosides bearing different aromatics and alkyls as S-substituents, and bearing glucose, mannose and galactose as glycosyl units. The process has been extended to a lactose derived thioglycoside and to a glucose derived sulfenamide. The process was chemo- and stereoselective, and X-ray analysis confirmed the structure and provided stereochemical information on the configuration at the sulfur atom. A model for the stereochemical outcome is proposed based on the steric environment of the sulfide.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...