Modular synthesis of oligoacetylacetones via site-selective silylation of acetylacetone derivatives†
Abstract
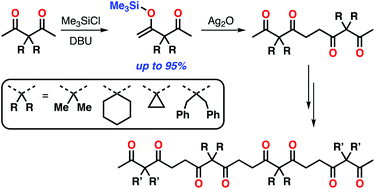
Oligoacetylacetones consisting of 3,3-disubstituted pentane-2,4-diones were synthesized through a terminal silylation and oxidative coupling protocol. Highly selective formation of mono-enol silyl ethers of 3,3-disubstituted acetylacetones was achieved using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as a base. Subsequent silver(I) oxide mediated coupling reactions provided tetraketones. Unique substituent dependence was found for the terminal-selective silylation of tetraketones. Finally, octaketones (tetramers of acetylacetone derivatives) with three types of monomer sequences were prepared in their discrete forms. Single crystal X-ray analysis revealed that the solid-state conformations of oligoketone chains were predominantly governed by the ketone sequence rather than substituents. However, differences in the packing structures induced by alkyl substituents led to significant differences in melting points for the structural isomers of octaketones.


 Please wait while we load your content...
Please wait while we load your content...