The palladium-catalyzed direct C3-cyanation of indoles using acetonitrile as the cyanide source†
Abstract
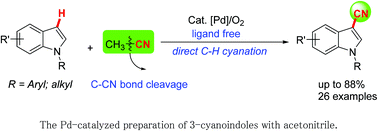
The ligand-free palladium-catalyzed C3-cyanation of indoles via direct C–H functionalization was achieved. This protocol, utilizing CH3CN as a green and readily available cyanide source, produced the desired products in moderate to good yields through transition-metal-catalyzed C–CN bond cleavage.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...