Synthesis of phenanthridines by I2-mediated sp3 C–H amination†
Abstract
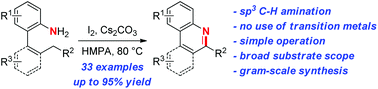
An I2-mediated synthesis of phenanthridines via intramolecular sp3 C–H amination of readily accessible aniline precursors is reported. The present synthetic process is straightforward and applicable to a broad variety of unprotected aniline substrates, and provides facile and efficient access to phenanthridine derivatives. This C–H amination protocol does not use transition metals, is operationally simple, and can be achieved on a gram scale.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...