Mono-functionalized derivatives and revised configurational assignment of amide naphthotubes†
Abstract
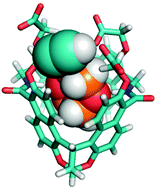
Amide naphthotubes with four carboxylate sidechains have been reported by us for selectively recognizing hydrophilic molecules in water and they have found applications in sensing and self-assembly. Modification of these macrocycles on the sidechains would further expand their applicability. Herein, we report the synthesis of mono-functionalized amide naphthotubes with one alkyne and three carboxylate groups. These naphthotubes show rather different binding affinities from that of the amide naphthotubes with four carboxylate sidechains. The partial self-inclusion of the alkyne group in the cavity was invoked to explain these differences. In addition, the syn- and anti-configurational isomers of the naphthotubes with four carboxylate groups were found to be assigned incorrectly in our earlier publication. Further evidence is provided here for the new assignment. The implications of this new structural assignment for the conclusions drawn in the earlier works are discussed as well.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...