Carbazole based Electron Donor Acceptor (EDA) catalysis for the synthesis of biaryl and aryl–heteroaryl compounds†
Abstract
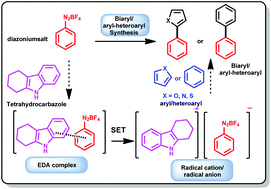
A highly regioselective, carbazole based Electron Donor Acceptor (EDA) catalyzed synthesis of biaryl and aryl–heteroaryl compounds is described. Various indole and carbazole derivatives were screened for the Homolytic Aromatic Substitution (HAS) reaction. Tetrahydrocarbazole (THC) was very efficient for the HAS transformation and proceeded via a complex formation between diazonium salt and electron rich tetrahydrocarbazole. The UV-Vis spectroscopy technique has been used to confirm the complex formation. The in situ generated EDA complex even in a catalytic amount is found to be efficient for the Single Electron Transfer (SET) process without any photoactivation. Biaryl compounds, 2-phenylfuran, 2-phenylthiophene, and 2-phenylpyrrole and bioactive compounds such as dantrolene and canagliflozin have been synthesized in moderate to excellent yields.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...