Synthesis of orthogonally protected and functionalized bacillosamines†
Abstract
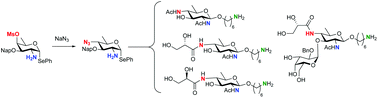
2,4-Diamino-2,4,6-trideoxyglucose (bacillosamine) is a monosaccharide found in many pathogenic bacteria, variation in the functionalities appended to the amino groups occurs depending on the species the sugar is derived from. We here report the first synthesis of bacillosamine synthons that allow for the incorporation of two different functionalities at the C-2-N-acetyl and C-4-amines. We have developed chemistry to assemble a set of conjugation ready Neisseria meningitidis C-2-N-acetyl bacillosamine saccharides, carrying either an acetyl or (R)- or (S)-glyceroyl at the C-4 amine. The glyceroyl bacillosamines have been further extended at the C-3-OH with an α-D-galactopyranose to provide structures that occur as post-translational modifications of N. meningitidis PilE proteins, which make up the bacterial pili.
- This article is part of the themed collection: Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...