β-Carboline directed regioselective hydroxylation by employing Cu(OAc)2 and mechanistic investigation by ESI-MS†
Abstract
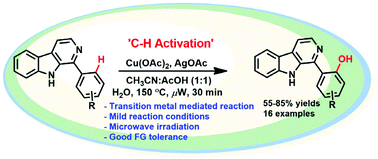
A β-carboline directed regioselective C–H activation protocol for hydroxylation has been developed by employing Cu(OAc)2, a cost-effective 3d metal. This fastidious reaction proceeds under microwave irradiation and furnishes exclusively ortho-hydroxylated products with moderate to good yields under greener reaction conditions. Gratifyingly, this approach retains considerable functional group tolerance and is feasible on both electron-rich and electron-deficient substrates. This protocol signifies monohydroxylation on β-carboline via C–H functionalization which leads into development of biologically relevant molecules. The reaction mechanism was confirmed by intercepting and characterizing all the proposed intermediates by ESI-QTOF-MS.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...