Zinc triflate catalyzed 1,3-indolylation of cyclohexanones: tandem condensation, dehydrogenation and aromatization sequence†
Abstract
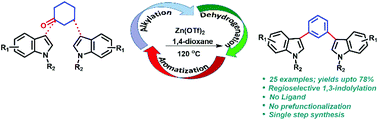
1,3-Bis(1-alkyl-1H-indol-3-yl)benzene derivatives have been synthesized through a Zn(OTf)2 catalyzed reaction between cyclohexanones and indoles. Cyclohexanones act as an aryl source via an alkylation–dehydrogenation–aromatization sequence, and undergo regioselective C–C bond formation with indoles at the 1,3-position. A useful library of these derivatives obtained in moderate to high yields has been prepared and a tentative mechanism has been proposed based upon GCMS analysis and time dependent NMR studies.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...