Iron(iii) chloride-promoted cyclization of α,β-alkynic tosylhydrazones with diselenides: synthesis of 4-(arylselanyl)-1H-pyrazoles†
Abstract
A highly efficient iron(III) chloride-promoted cyclization between α,β-alkynic tosylhydrazones and diselenides to form a 4-(arylselanyl)-1H-pyrazole skeleton is studied. This reaction forms C–N and C–Se bonds in one step by utilizing inexpensive iron(III) chloride instead of expensive transition metal additives. This strategy features easily synthesized substrates, mild reaction conditions and high tolerance to functional groups.
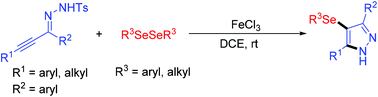
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...