Catalytic activities of cocaine hydrolases against the most toxic cocaine metabolite norcocaethylene
Abstract
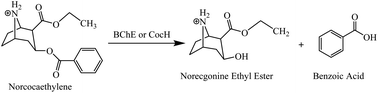
A majority of cocaine users also consume alcohol. The concurrent use of cocaine and alcohol produces the pharmacologically active metabolites cocaethylene and norcocaethylene, in addition to norcocaine. Both cocaethylene and norcocaethylene are more toxic than cocaine itself. Hence, a truly valuable cocaine-metabolizing enzyme for cocaine abuse/overdose treatment should be effective for the hydrolysis of not only cocaine, but also its metabolites norcocaine, cocaethylene, and norcocaethylene. However, there has been no report on enzymes capable of hydrolyzing norcocaethylene (the most toxic metabolite of cocaine). The catalytic efficiency parameters (kcat and KM) of human butyrylcholinesterase (BChE) and two mutants (known as cocaine hydrolases E14-3 and E12-7) against norcocaethylene have been characterized in the present study for the first time, and they are compared with those against cocaine. According to the obtained kinetic data, wild-type human BChE showed a similar catalytic efficiency against norcocaethylene (kcat = 9.5 min−1, KM = 11.7 μM, and kcat/KM = 8.12 × 105 M−1 min−1) to that against (−)-cocaine (kcat = 4.1 min−1, KM = 4.5 μM, and kcat/KM = 9.1 × 105 M−1 min−1). E14-3 and E12-7 showed an improved catalytic activity against norcocaethylene compared to wild-type BChE. E12-7 showed a 39-fold improved catalytic efficiency against norcocaethylene (kcat = 210 min−1, KM = 6.6 μM, and kcat/KM = 3.18 × 107 M−1 min−1). It has been demonstrated that E12-7 as an exogenous enzyme can efficiently metabolize norcocaethylene in rats.

 Please wait while we load your content...
Please wait while we load your content...