First enantioselective synthesis of gingesulfonic acids and unequivocal determination of their absolute stereochemistry†
Abstract
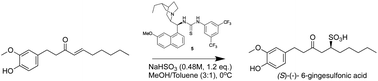
Herein we report the first organocatalysed enantioselective synthesis of gingesulfonic acids and shogasulfonic acids via a mild and convenient aminothiourea-catalysed conjugate addition of bisulfite to the olefin moiety of α,β-unsaturated carbonyls—a technology previously reported by us. A series of optically active naturally occurring sulfonic acids are prepared in their natural and unnatural configurations, and their absolute configurations are unequivocally confirmed by single crystal X-ray diffractometry.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...