Serendipitous base catalysed condensation–heteroannulation of iminoesters: a regioselective route to the synthesis of 4,6-disubstituted 5-azaindoles†
Abstract
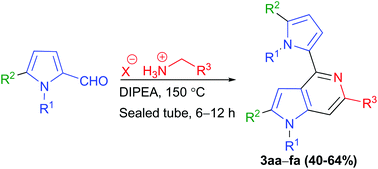
A serendipitous discovery of a novel one-pot synthesis of 4,6-disubstituted 5-azaindoles is reported herein. In the presence of Hunig's base, various N-substituted pyrrole-2-carboxaldehydes have been efficiently transformed into their corresponding 4,6-disubstituted 5-azaindoles through an imine mediated cascade condensation–heteroannulation. The synthetic value of the methodology is established by preparing a novel chemical analogue of a cannabinoid receptor type 2 (CB2) agonist.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...