Visible-light-promoted radical cross-coupling of para-quinone methides with N-substituted anilines: an efficient approach to 2,2-diarylethylamines†
Abstract
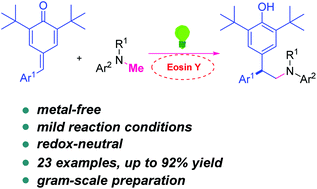
An efficient protocol to access 2,2-diarylethylamines via visible-light-promoted radical reactions of para-quinone methides (p-QMs) with N-alkyl anilines has been disclosed. These reactions feature metal-free, redox-neutral, and mild reaction conditions with wide functional group compatibility.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...