Multi-state amine sensing by electron transfers in a BODIPY probe†
Abstract
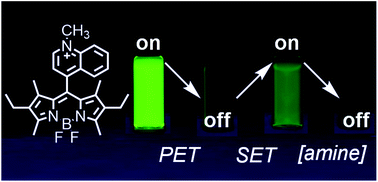
Amines are ubiquitous in the chemical industry and are present in a wide range of biological processes, motivating the development of amine-sensitive sensors. There are many turn-on amine sensors, however there are no examples of turn-on sensors that utilize the amine's ability to react by single electron transfer (SET). We investigated a new turn-on amine probe with a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) fluorophore. BODIPY fluorescence is first preprogrammed into an off state by internal photoinduced electron transfer (PET) to an electron-deficient quinolinium ring, resulting in fluorescence quenching. At low concentrations of aliphatic amine (0 to 10 mM), this PET pathway is shut down by external SET from the amine to the photoexcited charge-transfer state of the probe and the fluorescence is turned on. At high concentrations of amine (50 mM to 1 M), we observed collisional quenching of the BODIPY fluorescence. The probe is selective for aliphatic amines over aromatic amines, and aliphatic thiols or alcohols. The three molecular processes modulate the BODIPY fluorescence in a multi-mechanistic way with two of them producing a direct response to amine concentrations. The totality of the three molecular processes produced the first example of a multi-state and dose-responsive amine sensor.


 Please wait while we load your content...
Please wait while we load your content...
