Fast construction of isoquinolin-1(2H)-ones by direct intramolecular C–H/N–H functionalization under metal-free conditions†
Abstract
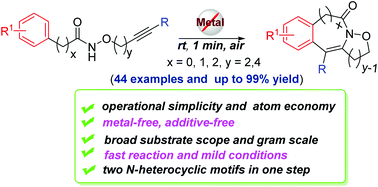
The general protocol for the synthesis of isoxazolidine-fused isoquinolin-1(2H)-ones was established with the help of bench stable hypervalent iodine reagent PIDA. Polycyclic six-, seven- and eight-membered N-heterocycles can be rapidly synthesized from available amides under metal-free conditions within 1 min at room temperature through C–H/N–H functionalization. Moreover, the protocol has the merits of broad substrate scope, atom economy and operational simplicity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...