Molecular replication using covalent base-pairs with traceless linkers†
Abstract
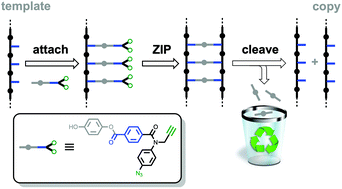
A unique feature of kinetically inert covalent base-pairing is that the nature of the chemical information that is transferred can be modulated by changing the chemical connectivity between the two bases. Formation of esters between phenols and benzoic acids has been used as a base-pairing strategy for sequence information transfer in template-directed synthesis of linear oligomers, but the copy strand produced by this process has the complementary sequence to the template strand. It is possible to form a base-pair between two benzoic acids by using a hydroquinone linker, which is eliminated when the product duplex is hydrolysed. Using this approach, covalent template-directed synthesis was carried out using a benzoic acid 3-mer template to produce an identical copy. This direct replication process was used in iterative rounds of replication leading to an increase of the population of the copied oligomer.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...