A facile method for the synthesis of dihydroquinoline-azide from the Lewis acid-catalyzed reaction of alkylidenecyclopropanes with TMSN3†
Abstract
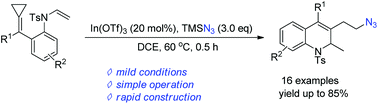
A facile synthetic method for the formation of dihydroquinoline-azide from alkylidenecyclopropanes and TMSN3 under the catalysis of a Lewis acid has been developed, and a number of azide-containing compounds can be instantly accessed in moderate to good yields. A click reaction with these azido compounds was also realized along with a mechanistic investigation.


 Please wait while we load your content...
Please wait while we load your content...