Thio radical-induced denitrogenative annulation of 1-azido-2-isocyanoarenes to construct 2-thiolated benzimidazoles†
Abstract
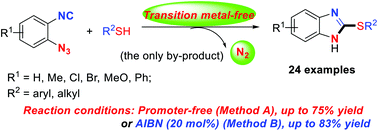
A method for the synthesis of 2-thiolated benzimidazoles is described starting from thiols and 1-azido-2-isocyanoarenes. The isocyano group works as an acceptor of various thio radicals, followed by denitrogenative annulation of the resulting imidoyl radical intermediates to the azido group, with nitrogen loss as the only process involving high bond-forming efficiency. The one-pot method for the synthesis of these products with high functional group tolerance in the benzimidazole-based ring is not available in previous literature.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...