Thiamine hydrochloride as a recyclable organocatalyst for the synthesis of bis(indolyl)methanes, tris(indolyl)methanes, 3,3-di(indol-3-yl)indolin-2-ones and biscoumarins†
Abstract
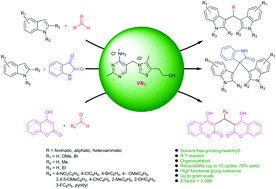
Thiamine hydrochloride was identified as an eco-friendly organocatalyst for the synthesis of a broad range of bis(indolyl)methanes in good to excellent yields. Green chemistry matrix calculations showed high atom economy and a small E-factor for the reaction. Moreover, the simple and easy operational protocol using a small amount of thiamine hydrochloride (1 mol%) makes this procedure an alternative approach for this transformation industrially. This protocol was also extended to the synthesis of naturally occurring alkaloids such as tris(indolyl)methane, arundine, vibrindole A, 3,3-di(indol-3-yl)indolin-2-ones and biscoumarin derivatives.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...