PEG-modified aziridines for stereoselective synthesis of water-soluble fulleropyrrolidines†
Abstract
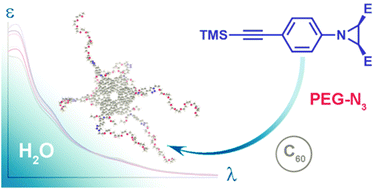
Diastereoselective synthesis of water-soluble fullerene compounds bearing a pharmacophore pyrrolofullerene-2′,5′-dicarboxylate unit is reported. The stereocontrol of the product configuration is achieved through stereospecificity of two consecutive concerted reactions: electrocyclic aziridine ring opening followed by 1,3-dipolar cycloaddition of the resulting azomethyne ylide. The solubility in water (up to 20 μM through direct dissolution) is secured by introducing a polyethylene glycol (PEG) hydrophilic pendant. The structure and molecular-mass distribution of the resulting PEGylated fulleropyrrolidines are exhaustively characterized by 1H, 13C NMR and HRMS. According to absorbance spectroscopy, AFM and DLS studies, the synthesized compound tends to aggregate in aqueous media forming associates of ca. 4–9 nm radius surrounded by a solvation shell resulting in an effective hydrodynamic diameter of ca. 90 nm. In view of notable solubility in water, well-defined chemical structure and resemblance to the compounds with known anti-HIV activity, the synthesized PEGylated diethyl trans-pyrrolofullerene-2′,5′-dicarboxylate might be an attractive candidate for biological evaluation.


 Please wait while we load your content...
Please wait while we load your content...