Facile synthesis of stable selenocystine peptides and their solution state NMR studies†
Abstract
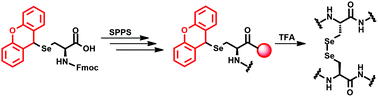
A facile general route for the synthesis of various selenocystine tripeptides containing acidic, basic and neutral side chain amino acids is reported. Here, TFA labile side chain protected selenocysteine has been used as a precursor for the synthesis of selenopeptides. The peptides are highly stable in dimethyl sulphoxide, thus enabling detailed NMR studies by solution phase 1- and 2-dimensional NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...