Hypervalent iodane mediated reactions of N-acetyl enamines for the synthesis of oxazoles and imidazoles†
Abstract
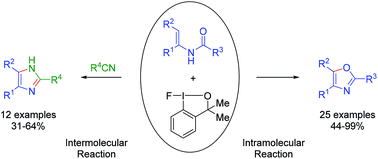
A hypervalent iodane reagent used for the intramolecular cyclization of N-acetyl enamines and intermolecular cyclocondensation of enamines and nitriles was investigated. The reaction was performed under mild conditions and gave oxazoles and imidazoles, respectively, in moderate to excellent yields. This transformation exhibits good reactivity, selectivity and functional group tolerance. The selectivity of the intra- or intermolecular reaction is dependent on the structure of N-acetyl enamines.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...