Tandem arylation and regioselective allylic etherification of 2,3-allenol via Pd/B cooperative catalysis†
Abstract
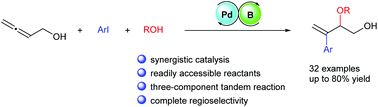
An efficient method for the construction of arylated allylic ethers was developed via three-component tandem arylation and allylic etherification of 2,3-allenol with aryl iodides and alcohols. In the cooperative catalytic system of a palladium complex and triethylborane, the process allows rapid access to functionalized 1-arylvinylated 1,2-diol derivatives in good to high yields with complete branch-selectivities. The synthetic utility of the present process was demonstrated by the late-stage functionalization of a drug molecule, the gram-scale synthesis and the elaboration of the products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...