Functionalization of activated methylene C–H bonds with nitroarenes and sulfur for the synthesis of thioamides†
Abstract
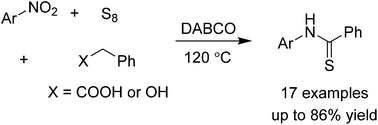
We report a method to obtain arylthioamides by the functionalization of sp3 C–H bonds in phenylacetic acids and benzyl alcohols. Reactions proceeded without the use of any solvents and were compatible with many functionalities and heterocycles. These conditions allow for a rapid synthesis of thioamides from simple, commercial substrates.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...