An enzymatic Finkelstein reaction: fluorinase catalyses direct halogen exchange†
Abstract
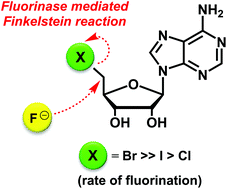
The fluorinase enzyme from Streptomyces cattleya is shown to catalyse a direct displacement of bromide and iodide by fluoride ion from 5′-bromodeoxyadenosine (5′-BrDA) and 5′-iododeoxyadenosine (5′-IDA) respectively to form 5′-fluorodeoxyadenosine (5′-FDA) in the absence of L-methionine (L-Met) or S-adenosyl-L-methionine (SAM). 5′-BrDA is the most efficient substrate for this enzyme catalysed Finkelstein reaction.


 Please wait while we load your content...
Please wait while we load your content...