Highly efficient asymmetric bioreduction of 1-aryl-2-(azaaryl)ethanones. Chemoenzymatic synthesis of lanicemine†
Abstract
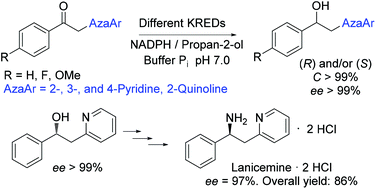
Different ketoreductases (KREDs) have been used to promote a highly selective reduction of several 1-aryl-2-(azaaryl)ethanones (azaaryl = pyridinyl, quinolin-2-yl), the corresponding secondary alcohols being obtained with very high yields and enantiomeric excesses (ee > 99%). The absolute configuration of each optically active alcohol has been assigned by means of modified Mosher and Kelly methods, two shielding effects being evaluated: (1) the Mosher phenyl ring effect on the azaaryl protons and (2) the one of the azaaryl ring on the Mosher methoxy group. In addition, the biologically active amine lanicemine has been synthesized from (R)-1-phenyl-2-(pyridin-2-yl)ethanol, thus proving the utility of the secondary alcohols here prepared.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...