Transition metal-free α-methylation of 1,8-naphthyridine derivatives using DMSO as methylation reagent†
Abstract
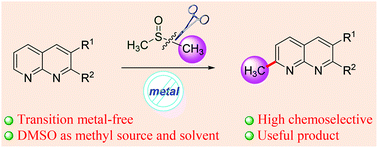
A practical approach to the direct α-methylation of 1,8-naphthyridines under mild reaction conditions has been developed using simple and readily available DMSO as a convenient and environmentally friendly carbon source. This method is transition metal-free and highly chemoselective, shows good functional group tolerance, and uses DMSO as a methyl source, providing efficient and rapid access to an important compound class, 2-methyl-1,8-naphthyridines.


 Please wait while we load your content...
Please wait while we load your content...