Iridium-catalyzed diastereoselective amination of alcohols with chiral tert-butanesulfinamide by the use of a borrowing hydrogen methodology†
Abstract
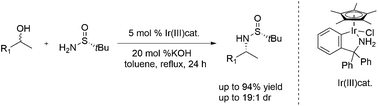
An iridium-catalyzed diastereoselective amination of alcohols with chiral tert-butanesulfinamide was developed under basic conditions, affording the optically active secondary sulfinamides in high yields and diastereoselectivities. The removal of the sulfinyl group from sulfonamides allowed a facile access to a wide range of α-chiral primary amines. This synthetic strategy was further applied in the synthesis of the marketed pharmaceuticals (S)-rivastigmine and NPS R-568.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...