Hyperconjomer stereocontrol of cationic polyene cyclisations†
Abstract
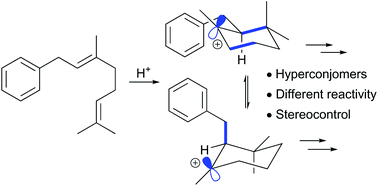
Polyene cyclisations are a powerful method for the direct generation of molecular complexity. This paper describes the use of computational methods to investigate the stereoselectivity of cationic polyene cyclisations of geranylbenzene derivatives. The outcomes highlight the different reactivity of hyperconjomers during the key Friedel–Crafts alkylation step, and informed a successful strategy for the synthesis of (±)-taiwaniaquinone G with improved levels of stereoselectivity.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...