An unprecedented oxidised julolidine-BODIPY conjugate and its application in real-time ratiometric fluorescence sensing of sulfite†
Abstract
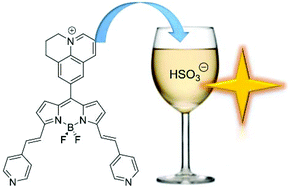
Reaction of a julolidine-based BODIPY compound with silver(I) ions in the presence of white light produced the oxidised julolidine version (OXJUL) containing a quaternary nitrogen. The oxidation of one ring at the julolidine site is highly unusual and there is no other reported literature example. The fluorescence maximum of OXJUL is altered from 648 nm to 608 nm by the addition of an aqueous solution of Na2SO3 over several minutes. In the presence of a large excess of sulfite a further slower reaction is observed which further shifts the emission maximum to 544 nm. The alterations form the basis of a real-time ratiometric sensor for sulfite and its detection in a white wine.


 Please wait while we load your content...
Please wait while we load your content...